How to do a case study
How to do a case study
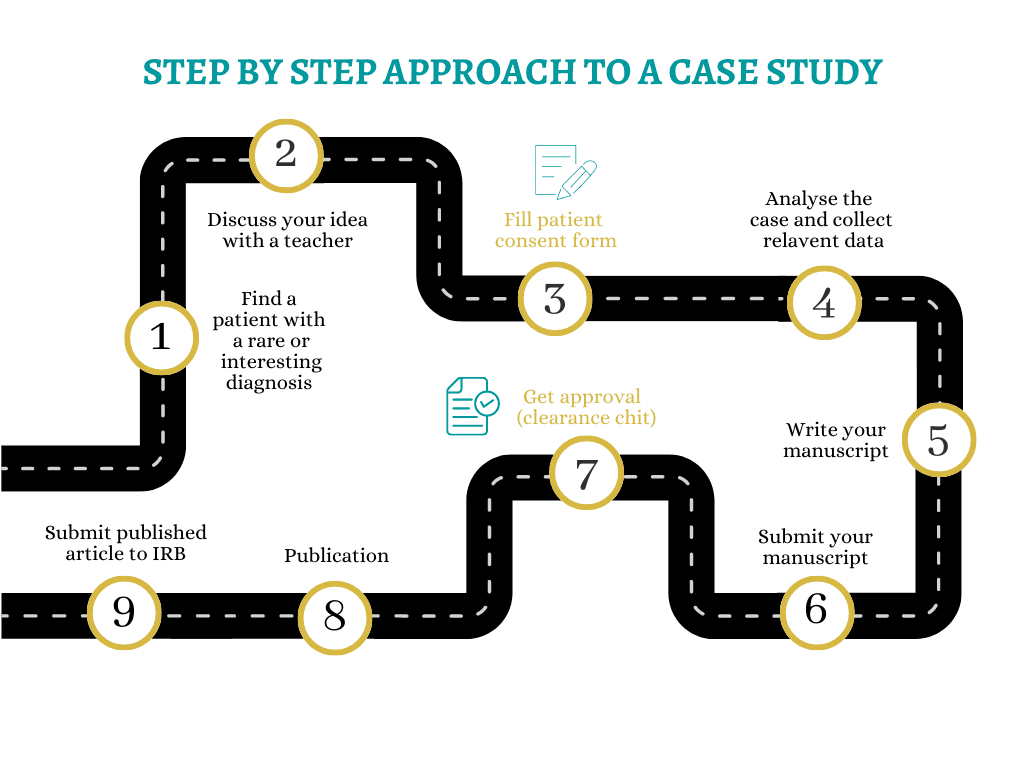


1
Select Interesting/Rare Patient;
Select Interesting/Rare Patient:
The first step is to choose a patient that is either interesting or rare in some way. This could be a patient with an unusual or rare medical condition, a patient with a complex treatment plan or a patient having unique response to treatment.
The first step is to choose a patient that is either interesting or rare in some way. This could be a patient with an unusual or rare medical condition, a patient with a complex treatment plan or a patient having unique response to treatment.
2
Discuss with teachers;
Discuss with teachers:
Discuss the research with your teacher / mentor to ensure that you are on the right track, also to get their guidance on what needs to be done and important information that needs to be collected.
Discuss the research with your teacher / mentor to ensure that you are on the right track, also to get their guidance on what needs to be done and important information that needs to be collected.
3
Get Patient Consent;
Get Patient Consent:
Before beginning any work on the case study, obtain consent from the patient (or their legal guardian) after explaining the process(informed consent). This is to ensure that the patient understands what you are doing and provide you with permission to use their medical information in your case study.
Download consent form template from this link.
Before beginning any work on the case study, obtain consent from the patient (or their legal guardian) after explaining the process(informed consent). This is to ensure that the patient understands what you are doing and provide you with permission to use their medical information in your case study.
Download consent form template from this link.
4
Analysis of Patient and collect relevant articles :
Analysis of Patient and collect relevant articles:
After getting approval from IRB you can go ahead and start analyzing the patient's medical history, current condition, treatment plan, and their response to treatment. You should also collect any relevant lab results, imaging studies, or other diagnostic tests that may help to understand the patient's condition. Make sure to remove all the patient personal information from the collected data before submitting. Afterwards, collect relevant articles, latest medical findings and review existing literature on the topic.
After getting approval from IRB you can go ahead and start analyzing the patient's medical history, current condition, treatment plan, and their response to treatment. You should also collect any relevant lab results, imaging studies, or other diagnostic tests that may help to understand the patient's condition. Make sure to remove all the patient personal information from the collected data before submitting. Afterwards, collect relevant articles, latest medical findings and review existing literature on the topic.
5
Write Manuscript;
Write Manuscript:
With all the information available, start writing the manuscript
With all the information available, start writing the manuscript
6
Submit your Manuscript;
Submit your Manuscript:
After completing the manuscript, you should email it to ethicsmisconduct4grsmuscience@gmail.com (email of IRB for clearance from the Ethics and Scientific Misconduct wing). Ethics and Scientific Misconduct wing of IRB will be checking for mainly 3 parts in your research: Plagiarism, Falsification and Fabrication.
After completing the manuscript, you should email it to ethicsmisconduct4grsmuscience@gmail.com (email of IRB for clearance from the Ethics and Scientific Misconduct wing). Ethics and Scientific Misconduct wing of IRB will be checking for mainly 3 parts in your research: Plagiarism, Falsification and Fabrication.
7
Get Clearance from IRB;
Get Clearance from IRB;
After the IRB has reviewed the manuscript, you will receive a feedback and be notified whether the manuscript is cleared for submission. If it is not, IRB will be informing on what changes that needs to be done to get the clearance.
After the IRB has reviewed the manuscript, you will receive a feedback and be notified whether the manuscript is cleared for submission. If it is not, IRB will be informing on what changes that needs to be done to get the clearance.
8
Manuscript ready for Publication;
Manuscript ready for Publication;
If the manuscript is cleared from Ethics and Scientific Misconduct wing of IRB, it can now be submitted to conferences or published in journals
If the manuscript is cleared from Ethics and Scientific Misconduct wing of IRB, it can now be submitted to conferences or published in journals
9
Submit Published Articles to IRB;
Submit Published Articles to IRB;
After the manuscript is published on a journal, you should submit the published article to the documentation wing of IRB to ensure that the article is documented as "published", so that it can be recorded in your personal student report. (This report can be requested by the student, from the IRB if needed.)
After the manuscript is published on a journal, you should submit the published article to the documentation wing of IRB to ensure that the article is documented as "published", so that it can be recorded in your personal student report. (This report can be requested by the student, from the IRB if needed.)
